This website is intended for U.S. healthcare professionals only.
If you are a patient, always seek the advice of a doctor or other qualified health professional regarding any questions you have about a medical condition. Hydroxyurea is a cytotoxic drug and must be handled with care.
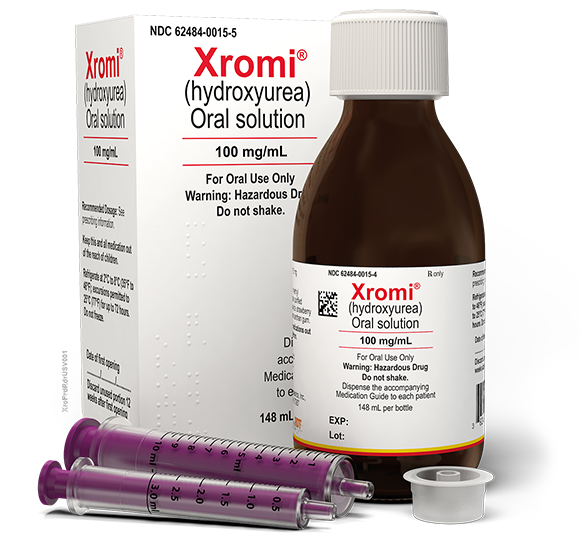
ABOUT XROMI
Take control before sickle cell does
with the only FDA-approved hydroxyurea oral solution
Accessible
Available at retail pharmacies in a consistent formulation1
Tolerability
Most common adverse reactions (incidence >5%) are neutropenia, thrombocytopenia, and papular rash2
Long shelf life
Refrigerated* shelf life of 2 years (12 weeks after first opening)1,2
Precise dosing
Delivers a personalized dose, accurate to within 10 mg2
Palatable
Strawberry flavor designed for children1,2
Indicated in children
For pediatric patients as young as 6 months old to help reduce the frequency of painful crises and the need for blood transfusions2
*Store at 2°C to 8°C (35°F to 46°F), excursions permitted to 25°C (77°F) for up to 72 hours.
XROMI is completely free from1,2:
Sugar • Lactose • Gluten • Alcohol
Helping to address adherence, dosing, and patient safety
- Liquid formulations provide dosing flexibility for use over a wide age range3
- Tablet crushing and splitting is associated with potential exposure to cytotoxic contamination4,5
- Compounded formulations take time to produce and may vary between bottles or over time6
- XROMI (hydroxyurea) oral solution must be handled with care when administered; anyone handling XROMI must follow applicable special handling and disposal procedures

REFERENCES
- Data on file. Rare Disease Therapeutics, Inc.
- Xromi (hydroxyurea) 100 mg/mL oral solution [package insert]. Franklin, TN: Rare Disease Therapeutics, Inc.; December 2024.
- Batchelor HK, Marriott JF. Formulations for children: problems and solutions. Special Issue: Br J Clin Pharmacol. 2015;79(3):405–418.
- United States Pharmacopeial Convention. USP general chapter <800> hazardous drugs – handling in healthcare settings, revision bulletin. December 1, 2019.
- United States Centers for Disease Control. NIOSH list of antineoplastic and other hazardous drugs in healthcare settings, 2016. https://www.cdc.gov/niosh/docs/2025-103/pdfs/2025-103.pdf?id=10.26616/NIOSHPUB2025103 Accessed April 14, 2025.
- Coache D, et al. Stability evaluation of compounded hydroxyurea 100 mg/mL oral liquids using a novel analytical method involving chemical derivatization. PLOS One. 2022;17(6):e0270206.
XROMI® is indicated to reduce the frequency of painful crises and reduce the need for blood transfusions in pediatric patients 6 months of age and older with sickle cell anemia with recurrent moderate to severe painful crises.
IMPORTANT SAFETY INFORMATION
|
WARNING: MYELOSUPPRESSION and MALIGNANCIES
|
CONTRAINDICATIONS
XROMI is contraindicated in patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of its formulation.
WARNINGS AND PRECAUTIONS
Myelosuppression
Hydroxyurea causes severe myelosuppression. Do not initiate treatment with hydroxyurea in patients if bone marrow function is markedly depressed. Bone marrow suppression may occur, and leukopenia is generally its first and most common manifestation. Thrombocytopenia and anemia occur less often and are seldom seen without a preceding leukopenia.
Some patients, treated at the recommended initial dose of 15 mg/kg/day, have experienced severe or life-threatening myelosuppression.
Evaluate hematologic status prior to and during treatment with XROMI. Provide supportive care and modify dose or discontinue XROMI as needed. Recovery from myelosuppression is usually rapid when therapy is interrupted.
Hemolytic Anemia
Cases of hemolytic anemia in patients treated with hydroxyurea for myeloproliferative diseases have been reported. In the setting of confirmed diagnosis of hemolytic anemia and in the absence of other causes, discontinue XROMI.
Malignancies
Hydroxyurea is a human carcinogen. In patients receiving long-term hydroxyurea for myeloproliferative disorders (a condition for which XROMI is not approved), secondary leukemia has been reported.
Secondary leukemia has also been reported in patients treated with long-term hydroxyurea for sickle cell anemia. Leukemia has also been reported in patients with sickle cell anemia and no prior history of treatment with hydroxyurea.
All patients using XROMI should be followed up on a long-term basis with regular blood counts to detect development of leukemia.
Skin cancer has also been reported in patients receiving long-term hydroxyurea. Advise protection from sun exposure and monitor for the development of secondary malignancies.
Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, XROMI can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus and to avoid becoming pregnant while being treated with XROMI.
Advise females of reproductive potential to use effective contraception during and after treatment with XROMI for at least 6 months after therapy. Advise males of reproductive potential to use effective contraception during and after treatment with XROMI for at least 1 year after therapy.
Vasculitic Toxicities
Cutaneous vasculitic toxicities, including vasculitic ulcerations and gangrene, have occurred in patients with myeloproliferative disorders during therapy with hydroxyurea. If cutaneous vasculitic ulcers occur, institute treatment and discontinue XROMI.
Live Vaccines
Avoid use of live vaccines in patients taking XROMI as it may potentiate the replication of the virus and/or may increase the adverse reaction of the vaccine because normal defense mechanisms may be suppressed by XROMI. Vaccination with live vaccines in a patient receiving XROMI may result in severe infection. Patient’s antibody response to vaccines may be decreased. Consider consultation with a specialist.
Risks with Concomitant Use of Antiretroviral Drugs
Pancreatitis, hepatotoxicity, and peripheral neuropathy have occurred when hydroxyurea was administered concomitantly with antiretroviral drugs, including didanosine and stavudine.
Macrocytosis
XROMI may cause macrocytosis, which is self-limiting, and is often seen early in the course of treatment. The morphologic change resembles pernicious anemia, but is not related to vitamin B12 or folic acid deficiency. This may mask the diagnosis of pernicious anemia. Prophylactic administration of folic acid is recommended.
Laboratory Test Interference
Interference with uric acid, urea, or lactic acid assays is possible, rendering falsely elevated results of these in patients treated with hydroxyurea.
Hydroxyurea may falsely elevate sensor glucose results from certain continuous glucose monitoring (CGM) systems and may lead to hypoglycemia if sensor glucose results are relied upon to dose insulin.
ADVERSE REACTIONS
Most common adverse reactions (incidence >5%) are neutropenia, thrombocytopenia, and papular rash.
USE IN SPECIFIC POPULATIONS
- Females and Males of Reproductive Potential: XROMI can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to initiating XROMI therapy. Advise females to immediately report pregnancy. XROMI may damage spermatozoa and testicular tissue, resulting in possible genetic abnormalities. Based on findings in animals and humans, male fertility may be compromised by treatment with XROMI.
- Pediatric Use: Continuous follow-up of the growth of treated children is recommended.
- Renal Impairment: Reduce dosage and closely monitor the hematologic parameters when XROMI is administered to patients with renal impairment who have a creatinine clearance of less than 60 mL/min.
- Hepatic Impairment: Close monitoring of hematologic parameters is advised in these patients receiving XROMI.
OVERDOSAGE
Acute mucocutaneous toxicity has been reported in patients receiving hydroxyurea at doses several times above the therapeutic dose. Soreness, violet erythema, oedema on palms and soles followed by scaling of hand and feet, severe generalized hyperpigmentation of the skin, and stomatitis have been observed.
Please see complete Prescribing Information including BOXED WARNING and Patient Information or at www.xromi.com/pi.
To report SUSPECTED ADVERSE REACTIONS, contact Rare Disease Therapeutics, Inc., at 1-844-472-7389 or by email at safety@raretx.com or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
XRO-ISIF-005
INDICATION & IMPORTANT SAFETY INFORMATION (including BOXED WARNING)
XROMI® is indicated to reduce the frequency of painful crises and reduce the need for blood transfusions in pediatric patients 6 months of age and older with sickle cell anemia with recurrent moderate to severe painful crises.
IMPORTANT SAFETY INFORMATION
|
WARNING: MYELOSUPPRESSION and MALIGNANCIES
|
CONTRAINDICATIONS
XROMI is contraindicated in patients who have demonstrated a previous hypersensitivity to hydroxyurea or any other component of its formulation.
WARNINGS AND PRECAUTIONS
Myelosuppression
Hydroxyurea causes severe myelosuppression. Do not initiate treatment with hydroxyurea in patients if bone marrow function is markedly depressed. Bone marrow suppression may occur, and leukopenia is generally its first and most common manifestation. Thrombocytopenia and anemia occur less often and are seldom seen without a preceding leukopenia.
Some patients, treated at the recommended initial dose of 15 mg/kg/day, have experienced severe or life-threatening myelosuppression.
Evaluate hematologic status prior to and during treatment with XROMI. Provide supportive care and modify dose or discontinue XROMI as needed. Recovery from myelosuppression is usually rapid when therapy is interrupted.
Hemolytic Anemia
Cases of hemolytic anemia in patients treated with hydroxyurea for myeloproliferative diseases have been reported. In the setting of confirmed diagnosis of hemolytic anemia and in the absence of other causes, discontinue XROMI.
Malignancies
Hydroxyurea is a human carcinogen. In patients receiving long-term hydroxyurea for myeloproliferative disorders (a condition for which XROMI is not approved), secondary leukemia has been reported.
Secondary leukemia has also been reported in patients treated with long-term hydroxyurea for sickle cell anemia. Leukemia has also been reported in patients with sickle cell anemia and no prior history of treatment with hydroxyurea.
All patients using XROMI should be followed up on a long-term basis with regular blood counts to detect development of leukemia.
Skin cancer has also been reported in patients receiving long-term hydroxyurea. Advise protection from sun exposure and monitor for the development of secondary malignancies.
Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, XROMI can cause fetal harm when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus and to avoid becoming pregnant while being treated with XROMI.
Advise females of reproductive potential to use effective contraception during and after treatment with XROMI for at least 6 months after therapy. Advise males of reproductive potential to use effective contraception during and after treatment with XROMI for at least 1 year after therapy.
Vasculitic Toxicities
Cutaneous vasculitic toxicities, including vasculitic ulcerations and gangrene, have occurred in patients with myeloproliferative disorders during therapy with hydroxyurea. If cutaneous vasculitic ulcers occur, institute treatment and discontinue XROMI.
Live Vaccines
Avoid use of live vaccines in patients taking XROMI as it may potentiate the replication of the virus and/or may increase the adverse reaction of the vaccine because normal defense mechanisms may be suppressed by XROMI. Vaccination with live vaccines in a patient receiving XROMI may result in severe infection. Patient’s antibody response to vaccines may be decreased. Consider consultation with a specialist.
Risks with Concomitant Use of Antiretroviral Drugs
Pancreatitis, hepatotoxicity, and peripheral neuropathy have occurred when hydroxyurea was administered concomitantly with antiretroviral drugs, including didanosine and stavudine.
Macrocytosis
XROMI may cause macrocytosis, which is self-limiting, and is often seen early in the course of treatment. The morphologic change resembles pernicious anemia, but is not related to vitamin B12 or folic acid deficiency. This may mask the diagnosis of pernicious anemia. Prophylactic administration of folic acid is recommended.
Laboratory Test Interference
Interference with uric acid, urea, or lactic acid assays is possible, rendering falsely elevated results of these in patients treated with hydroxyurea.
Hydroxyurea may falsely elevate sensor glucose results from certain continuous glucose monitoring (CGM) systems and may lead to hypoglycemia if sensor glucose results are relied upon to dose insulin.
ADVERSE REACTIONS
Most common adverse reactions (incidence >5%) are neutropenia, thrombocytopenia, and papular rash.
USE IN SPECIFIC POPULATIONS
- Females and Males of Reproductive Potential
XROMI can cause fetal harm when administered to a pregnant woman. Verify the pregnancy status of females of reproductive potential prior to initiating XROMI therapy. Advise females to immediately report pregnancy.
XROMI may damage spermatozoa and testicular tissue, resulting in possible genetic abnormalities. Based on findings in animals and humans, male fertility may be compromised by treatment with XROMI. - Pediatric Use: Continuous follow-up of the growth of treated children is recommended.
- Renal Impairment: Reduce dosage and closely monitor the hematologic parameters when XROMI is administered to patients with renal impairment who have a creatinine clearance of less than 60 mL/min.
- Hepatic Impairment: Close monitoring of hematologic parameters is advised in these patients receiving XROMI.
OVERDOSAGE
Acute mucocutaneous toxicity has been reported in patients receiving hydroxyurea at doses several times above the therapeutic dose. Soreness, violet erythema, oedema on palms and soles followed by scaling of hand and feet, severe generalized hyperpigmentation of the skin, and stomatitis have been observed.
Please see complete Prescribing Information including BOXED WARNING and Patient Information or at www.xromi.com/pi.
To report SUSPECTED ADVERSE REACTIONS, contact Rare Disease Therapeutics, Inc., at 1-844-472-7389 or by email at safety@raretx.com or contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
All images on this website are for illustrative purposes only and do not depict actual patients.
©2025 Rare Disease Therapeutics, Inc. All Rights Reserved.
XROMI® is a registered trademark of Nova Pharmaceuticals 2025-26. All Rights Reserved.
Intended for US Healthcare Professionals Only.
Privacy Policy & Terms of Use
XROweb010E January 2026


